
TITLE: Postdoctoral scholar
EMAIL: b4taylor@nullucla.edu
OFFICE: Boyer 570
EDUCATION:
B.S. (Biomedical Engineering) University of Virginia (2009)
Ph.D. (Bioengineering) University of California San Diego (2015)
Research Interests
NF-κB controls some of the most potent antiviral and antibacterial machinery that mammalian cells possess. It’s activated by hundreds of different signals, be they exogenous, endogenous, or synthetic. Tens of negative feedback regulators of NF-κB have been identified, which all serve to attenuate its activity. And while proper activation of NF-κB is a hallmark of a functional inflammatory response, improper activation is associated with pathologies in conditions ranging from Alzheimer’s to cancer to atherosclerosis.
We’ve seen tantalizing glimpses that NF-κB activates via intricately-controlled dynamics of translocation to and from the nucleus, and it’s been hypothesized that these patterns might convey information, allowing a cell to discriminate between different inputs using this single transcription factor. However, we’re still unclear on what these specific dynamic codes are, and how the information they convey might be decoded by the cell.
Using single-cell microscopy and automated tracking and measurement, I’m hoping to help crack the NF-κB dynamical code. I think it’s likely that NF-κB dynamics can convey information, not just about the nature of a particular stimulus, but about the disease state of a cell. Knowing what healthy and aberrant NF-κB dynamics look like (and even how to modulate one into the other) could afford new windows into both diagnosis and treatment.
Publications
Selimkhanov J*, Taylor B*, Yao J, et al. Accurate information transmission through dynamic biochemical signaling networks. Science. 2014; 346(6215):1370-1373.
Cheng Z*, Taylor B*, Ourthiague DR, Hoffmann A. Distinct single-cell signaling characteristics are conferred by the MyD88 and TRIF pathways during TLR4 activation. Sci Signal. 2015; 8(385):1-13.
(* denotes equal contribution)
Chemistry & Biochemistry Graduate Student (2009-)
Office: NSB 3318
Phone: 858-822-4673
B.S. Cellular & Molecular Biology, San Diego State University 2009
Research Interests
Coming soon.
Chemistry and Biochemistry Graduate Student (2009-)
 Office: NSB 4318
Office: NSB 4318
Phone: 858-822-4673
B.S. Chemistry, Boston University 2009
Research Interests
I am interested in the dynamic encoding of stimulus specificity in the canonical NFκB pathway.

TITLE: Bioinformatics and Systems Biology Graduate Student (2008-June 2014)
OFFICE: NSB 3209
PHONE: 520-4717076
EMAIL: mshokhir at ucsd.edu
EDUCATION:
B.S. Computer Science, Biochemistry and Molecular Biophysics, University of Arizona – 2008
LINKEDIN: http://www.linkedin.com/pub/maxim-shokhirev/19/948/62
Research Interests
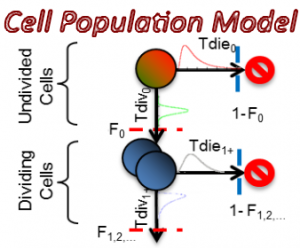

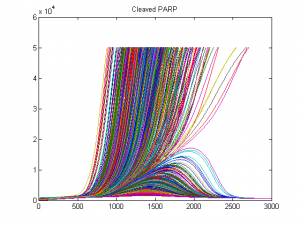
I am interested in understanding how cell population dynamics are coordinated on the cellular and biochemical levels. A prime example, the mammalian immune response involves seemingly stochastic cellular decisions to respond, divide, and undergo programmed cell death. Understand how immune cells make decisions that result in an effective population response sheds light on the nature of multi-cellular biology and may provide insights into treating immune disorders and cancers. To this end, I have developed computational tools for describing cell population dynamics in terms of stochastic cellular processes, cell tracking software for quantifying time-lapse microscopy movies, performed single-cell RNAseq experiments, ran immunfluorescence studies, and constructed kinetic ODE models which incorporate the cell-cycle, apoptosis, and NFkB signaling. This has allowed me to characterize the responses of primary mouse B cells under diverse conditions in a multi-scale manner.
Publications
- Shokhirev MN and Alexander Hoffmann (2013) FlowMax: a computational tool for maximum likelihood deconvolution of CFSE time courses. PLoS One.
- Jiao B, Ma H, Shokhirev MN, Drung A, Yang Q, et al. (2012) Paternal RLIM/Rnf12 is a survival factor for milk-producing alveolar cells. Cell 149: 630-641.
- Grubisic I, Shokhirev MN, Orzechowski M, Miyashita O, Tama F (2010) Biased coarse-grained molecular dynamics simulation approach for flexible fitting of X-ray structure into cryo electron microscopy maps. J Struct Biol 169: 95-105.
- Berry RE, Shokhirev MN, Ho AY, Yang F, Shokhireva TK, et al. (2009) Effect of mutation of carboxyl side-chain amino acids near the heme on the midpoint potentials and ligand binding constants of nitrophorin 2 and its NO, histamine, and imidazole complexes. J Am Chem Soc 131: 2313-2327.
- Knipp M, Yang F, Berry RE, Zhang H, Shokhirev MN, et al. (2007) Spectroscopic and functional characterization of nitrophorin 7 from the blood-feeding insect Rhodnius prolixus reveals an important role of its isoform-specific N-terminus for proper protein function. Biochemistry 46: 13254-13268.
- Berry RE, Shokhireva T, Filippov I, Shokhirev MN, Zhang H, et al. (2007) Effect of the N-terminus on heme cavity structure, ligand equilibrium, rate constants, and reduction potentials of nitrophorin 2 from Rhodnius prolixus. Biochemistry 46: 6830-6843.
- Borunda, P.; Brewer, C.; Erten, C.; King, N.; Nation Z.; Shokhirev, M. (2005) GSPIM: Graphical Visualization Tool for MIPS Assembly Programming and Simulation. SIGCSE 2005.
Chemistry and Biochemistry Graduate Student (2008-)
 dnrios@nullucsd.edu
dnrios@nullucsd.edu
Office: 858-822-4673
B.S. Biochemistry, California Polytechnic State University San Luis Obispo, 2008
Research Interests
I am interested in the dynamics of interferon beta production in response to TLR stimulation. I plan to examine, through experimental and computational techniques, the coordination of NF-kappaB and ISGF3 in mounting an innate immune response.
Bioinformatics Graduate Student (1967 -)
-)
ploriaux@nullbioinf.ucsd.edu
Office: NSB 4318
Phone: 858-822-4673
B.S. Bioengineering, Univ. of Washington 2001
M.S. Computer Science & Engineering, Univ. of California San Diego, 2011
Research Interests
My current research interests are in understanding the molecular mechanisms that process information in living cells. Cell systems represent a particular challenge in this regard in that their machinery is capable of operating over many orders of magnitude in both space and time. Computational modeling is particularly suited to making conjectures about how systems operate over a high dynamic range in these domains, and these conjectures can then be verified or refuted in the laboratory. Presently I am constructing a model of concurrent activation of the JNK and NF-kappa B signaling pathways, with the goal of understanding how the basal state of the system biases the cellular response towards one or the other of these two contradictory signals. The results could have implications to our basic understanding of why different cell types respond differently to the same set of inflammatory and apoptotic signals.
Publications
| Citation |
Link |
Of elections and cell-death decisions.
Loriaux P, Hoffmann A.
Mol Cell. 2009 May 15;34(3):257-8. |
PubMed |
A framework for modeling the relationship between cellular steady-state and stimulus-responsiveness.
Loriaux PM, Hoffmann A.
Methods Cell Biol. 2012;110:81-109. |
PubMed |
Characterizing the Relationship between Steady State and Response Using Analytical Expressions for the Steady States of Mass Action Models.
Loriaux PM, Tesler G, Hoffmann A.
PLoS Comput Biol. 2013 Feb;9(2):e1002901. |
PubMed |
A protein turnover signaling motif controls the stimulus-sensitivity of stress response pathways.
Loriaux PM, Hoffmann A.
PLoS Comput Biol. 2013 Feb;9(2):e1002932. |
PubMed |
Biomedical Sciences Graduate Student (2011-2013)
 j0huang@nullucsd.edu
j0huang@nullucsd.edu
Office: NSB 3320
Phone: 858-822-4673
B.S. Chemistry, Cornell, 2007
Research Interests
My research interests are to study the pleiotropic effects of drugs on the dynamic regulation of NFkB signaling system using both biochemical assays and single cell experiments. Using a systems pharmacology approach, the goal is to determine a combination of chemical perturbations which could yield specific target effects on gene expression.
Publications
| Citation |
Link |
Control of self-assembly of lithographically patternable block copolymer films.
Bosworth JK, Paik MY, Ruiz R, Schwartz EL, Huang JQ, et al
ACS Nano. 2008 Jul;2(7):1396-402. |
PubMed |
















